Q Lost Q Gained
Calorimetry is the measurement of the amount of heat gained or lost during some particular physical or chemical change. Heats of fusion or vaporization, heats of solution, and heats of reaction are examples of the kinds of determination that can be made in calorimetry. The term itself derives the Latin word for heat, caloric, as is the name of the instrument used to make these determinations, the calorimeter.
Chemistry: Heat Problems ANSWER KEY q=mc∆T q gained = q lost q = -q mc∆T = - mc∆T 1. The specific heat of ethanol is 2.46 J/goC. Find the heat energy required to raise the temperature of.
Calorimetry is the science associated with determining the changes in energy of a system by measuring the heat exchanged with the surroundings. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. In calorimetry it is often desirable to know the heat capacity of the calorimeter itself rather than the heat capacity of the entire calorimeter system (calorimeter and water). The heat (Q) released by a reaction or process is absorbed by the calorimeter and any substances in the calorimeter.
- Calculate the heat lost by the engine: Q. F,engine) = (300 kg) (0.45 kJ/kg˚C) (200˚C – 150˚C) = 6,750 kJ. This must equal the heat gained by the antifreeze (assume the final temperature of the antifreeze is also 150˚C): Q. Gained = Qlost = 6,750 kJ. Use the energy equation to determine the.
- Recall that Q is the amount of heat lost or gained. We want to know the final temperature of the system, and we have determined that occurs when the amount of heat transferred from the water to the bucket equals the amount of heat that is transferred from the bucket to the water.
Results & Discussions~
Table 6-A Determination of the amount of heat energy transferred using hot and cold water.
For this part of the experiment, we are comparing the heat gained and the heat lost by the system. Since it is in the calorimeter, we assume that the system is isolated. Which means that the heat gained is equal to the heat lost ( Q gained = Q lost ). Using the specific heat of water, C = (1 cal/g-Co) we compute the heat gained and the heat lost by using the equations: Q gained by the cold water = m cold water C (T final – T cold water ) and Q lost by the hot water = m hot water C (T hot water – T final ). All the necessary data are gained by measuring from the actual experiment. After some computation and doing these with three trials, we got a percent differences of 7.43 % , 0.71 % , and 4.26%.
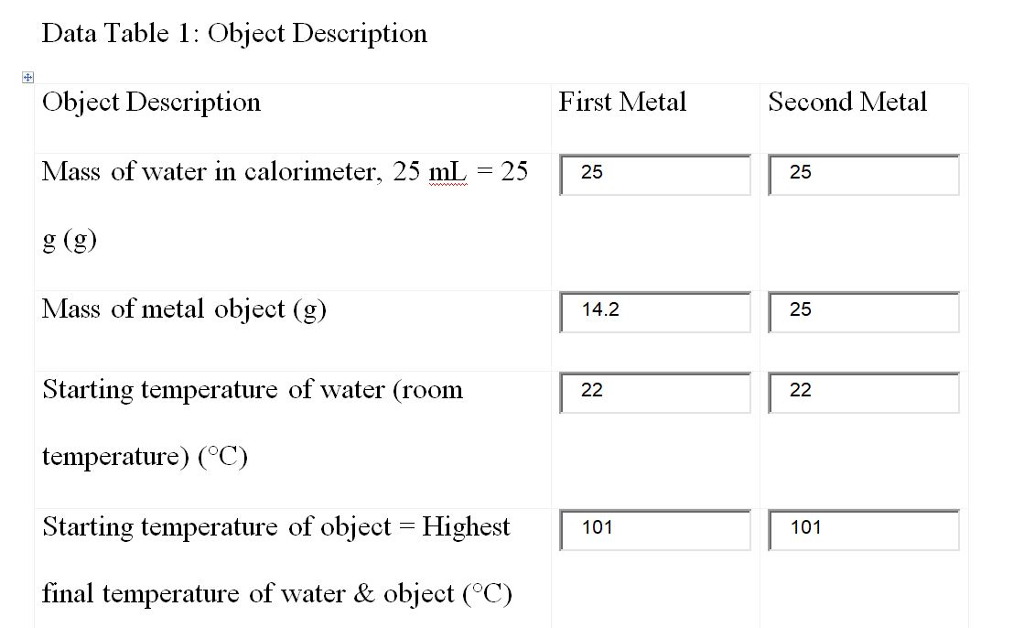
Table 6 – B : Determination the Specific Heat of Materials.
On the second part, we are getting the specific heat of the aluminum, copper, and lead. To do so, we are given a sample of the metals and we are getting the data from the steps that was stated in the manual, and when the necessary data are gained, we can susbstitute it to the equation : m metal ( C p metal ) ( T metal – T final ) = m water ( C p water ) ( T final – T cold water ). We still use C pwater = 1 cal/g-C o . As a result, we got 47.9 % , 92.25 % , and 58.67 % from the metals that we compared to the theoretical value of the specific heats. These are relatively high because of some factors like, the room temperature, measurement-error, human error , and other factors.
Q Gained Q Lost Heat Transfer
Summary~
To sum up, the amount of heat, Q, required raising the temperature of a solid body or material at constant pressure depends on the change in temperature ∆T of the material, its mass (m), and a characteristic of the material forming the body called its specific heat, c. This relationship is expressed by the equation Q = mc∆T and the dimensions of C are thus heat per unit mass per unit temperature change. We know that when two bodies, initially at different temperatures, are placed in intimate contact, in time they will come to equilibrium at some intermediate temperature. Provided no heat is lost to or gained from the surroundings, the quantity of heat lost by the hotter body is equal to that gained by the colder body. This is the process which occurs in the method of mixtures that you will used (Bauer, 2014).
In our experiment, adding hot water to cold water wasn’t the same result as add cold water to hot water because of the heat is transferred from high temperature to low temperature. Heating up a materials caused expanding, speeding up and pushing further apart of its molecules. Also hot water has more thermal energy before we were mixed them than after we mixed them and they conserved the energy because of the heat lost and gained are almost equal.
On the other hand, the specific heat capacity of any metals is lower compared to the specific heat capacity of water.
Conclusion~
First of all, calorimeter was not completely isolated and heat was lost also before the hot water was poured into calorimeter and the room temperature (air-conditioned) is the one which affects the values of our results. Meanwhile, in the experiment determining the specific heat of metals, we got higher percentage errors these are due to heat loss to the surroundings while we are trying to transfer the hot metal objects, boiling point of metals are too long, room temperature, and apparatuses. However we can conclude that the specific heat of metals is lower than the specific heat of water.
Problem Solving~
Number 1
Number 2


Q Gained Equals Q Lost
Number 3