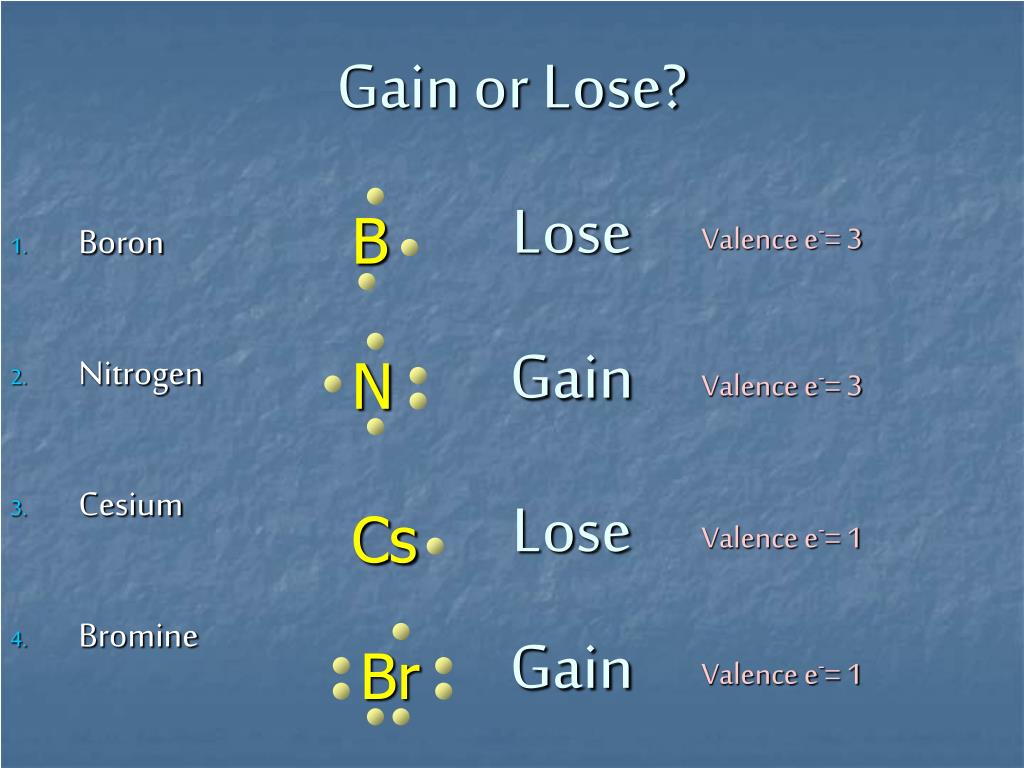Br Lose Or Gain Electrons
- Will Be Gain Or Lose Electrons When Forming An Ion
- Ba Gain Or Lose Electrons
- Will Bromine (br) Gain Or Lose Electrons To Form An Ion
- Losing And Gaining Electrons Worksheet
Nitrogen atoms gain 3 electrons and form the nitride ion, N3. Nitrogen atoms also form covalent bonds where they share 3 electrons and do not become ions. Bromine atoms gain 1 electron and form. Carbon has four electrons in it's valence, and possess the property of catenation. It forms covalent bonds, and hence, leads to the formation of different elements. It cannot lose or gain electrons, due to it's tetravalency. Hope this will help you.
How many electrons are there in #Br^-#?
Will Be Gain Or Lose Electrons When Forming An Ion

1 Answer
Explanation:
Ba Gain Or Lose Electrons
The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a
Below is the Lewis dot structure for a neutral bromine atom, which has seven valence electrons.
Below is the Lewis dot structure for a
Will Bromine (br) Gain Or Lose Electrons To Form An Ion
The diagram below shows how a bromine atom gains an electron from the element lithium in order to form the ionic compound LiBr.
Losing And Gaining Electrons Worksheet